Chemistry Chapter 6 Periodic Table
- Slides: 26
Download presentation

Chemistry Affiliate half dozen The Periodic Table and Periodic Law

6. 1 Development of the Modernistic Periodic Table § Objectives: § ane. Explain how elements are organized in a periodic tabular array § two. Identify primal features of the periodic table

How the Elements are Organized + Early on & Modern § Chemists used the properties of elements to sort them into groups § Dimitri Mendeleev was the first to arrange the elements in the periodic table in social club of increasing mass § In the modern periodic tabular array, elements are arranged in social club of increasing atomic number § http: //www. inorganicventures. com/extra south/pertable/

§ The design of properties within a catamenia repeats as you lot movement from ane catamenia to the next § Elements that have similar properties finish up in the same cavalcade in the table § PERIODIC Law: when elements are arranged in order of increasing diminutive number, there is a periodic repetition of their physical and chemical properties § Q: How many periods are there in a periodic table? § A: vii

Key Features: 3 Broad Classes § Groups with letter of the alphabet A are called representative elements § Table has iii categories: metals, non-metals, and metalloids § Metals to the left, non-metals to the right, and metalloids along the stair stride § Metalloids are too called semiconductors

§ Metals have: § Loftier conductivity § High luster (when clean) § Are ductile-drawn into wires § Are malleable § Not-metals: § Poor conductors § nonlustrous

§ Metalloids (border the stairstep) generally have some properties of metals and some properties of nonmetals § Here'southward a preview of the tabular array: § 1 A metal elements known as alkali metals § 2 A metal elements known as element of group i earth metals § 7 A nonmetal elements known every bit halogens (halogens means "salt formers" § 0 nonmetal elements known as noble gases (also chosen group eight A)

§ On the flat of function of the table between groups three – 12 lie the group B elements § These are the transition elements which are divided into transition metals and inner transition metals § The inner transition metals are further divided into the lanthanide serial and actinide series § The inner transition metals are sometimes called the rare earth metals

6. two Classification of the Elements § Objectives: § i. Explain why elements in the aforementioned grouping have similar backdrop § 2. Identify the iv blocks of the periodic table based on their electron configuration

Classifying by Electron Configuration § Elements tin be sorted in noble gases, representative elements, transition metals or inner transition metals based on their electron configurations § Electrons exist in "shells" (like to orbits) around the nucleus

§ The first shell tin hold up to two electrons § The second shell tin can hold up to eight electrons & the 3rd up to xviii east- (count the number of elements across the menses) § If the shells are full, the atom is peculiarly stable § All elements in Group viii A, likewise chosen the 0 elements, have full outer shells § These are the noble gases and they are inert & nonreactive with other elements because of their total shells § The elements in the 8 A group have a charge of 0

§ Elements on the table are neutral, simply the groups have tendencies § All elements in group one A, the alkali metals, readily lose i electron to complete their outer beat out § If they lose that one electron, the 1 A element volition deport a charge of one+ § Grouping 2 A, the alkaline metal globe metals, readily loses 2 electrons § Group 2 A will comport a charge of 2+ § Grouping 3 A volition comport a charge of 3+ if it loses 3 electrons

§ Group 4 A has 2 possible charges: 4+ or 4 - because it tin either lose or gain 4 electrons to complete its outer shell § Group v A also has 2 possible charges: 5+ or 3§ Group half-dozen A carries a 2 - charge considering information technology gains 2 electrons to complete its outer beat § seven A (the halogens) has a 1 - charge § 8 A (the nobles) has a 0 charge because its outer shell is already full § The representative elements are located in the elevated part of the table and are group A
§ The transition metals, on the flat part of the table, are the B elements § Two main groups: transition metals and inner transition metals § Inner transition metals also known as the rare-globe elements § Many elements in the transition group can take variable charges

§ There are some general, more common charges these elements take § Virtually elements in this group have a accuse of 2+ except for the groups beginning with chromium (Cr, vi B) and copper (Cu, 1 B) § Elements in the Cr and Cu groups by and large have a 1+ charge § 80% of elements are metals, almost all solid at room temperature

§ Metals are located downstairs to the left on the table § Metalloids are located on either side of the stairs § Nonmetals are about 20% of the elements & are located upstairs to the correct with the exception of hydrogen § General rules: § 1. positive elements react with negative elements § 2. elements with full outer shells practise not react

The Four Blocks § The iv blocks are chosen south, p, d and f § This explains the odd shape of the periodic table § Each block represents an atom'due south energy sublevel being filled with valence electrons § S level holds upwardly to two e- (one orbital) § P level holds up to 6 eastward- (three orbitals) § D level holds up to 10 e- (five orbitals) § F level can hold up to 14 e- (vii orbitals)

vi. 3 Periodic Trends § Objectives: § 1. Compare period and group trends of several properties § 2. Chronicle menstruation and group trends in atomic radii to electron configuration

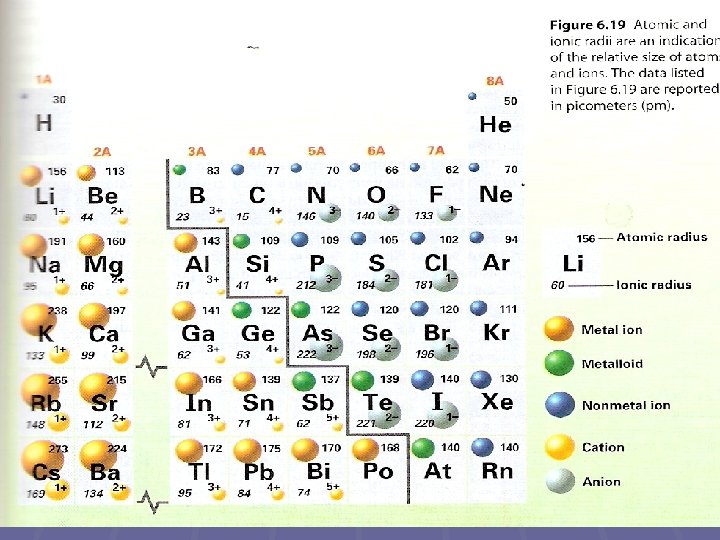
Trend in Atomic Size § Size is expressed as diminutive radius § Definition: atomic radius is one one-half the distance betwixt the nuclei of ii atoms of the same element when the atoms are joined § In full general, atomic size increases from meridian to bottom inside a grouping and decreases from left to right beyond a flow

Trend in Ions § Definition: an ion is an atom or group of atoms that has a positive or negative charge due to loss or gain of an electron § An atom is electrically neutral due to equal numbers of electrons and protons § Electrically neutral atoms are different from stable atoms § Stable atoms have full outer electron shells § Atoms strive to accept the stable configuration of the nobles

§ Positive and negative ions form when electrons are transferred between atoms § The transfer occurs in fashion that the charge will add up to nil § Definition: a cation is an ion with a positive accuse § Definition: an anion is an ion with a negative charge § Ionic radius from left to right beyond the period for positive ions gradually decreases § Get-go about grouping 5 A or 6 A the size of the much larger anions gradually decreases

Ionization Free energy, Size & Trends § Definition: the energy required to remove an electron from an atom is the ionization energy § This free energy is measured when the atom is in a gaseous land § Definition: the energy required to remove the showtime electron is the starting time ionization energy

§ First ionization energies tend to decrease from peak to bottom within a group and increase from left to right beyond a menses § SIZEWISE: Cations are always smaller than the atoms from which they formed (because they lost one or more electrons) § SIZEWISE: Anions are always larger than the atoms from which they form (because they gained one or more than electrons)

Trend in Electronegativity § Definition: electronegativity is the ability of an atom of an element to attract electrons when the cantlet is in a chemical compound § In general, nobles don't concenter electrons or form many compounds § Electronegativity is small-scale to zero for nobles § The nigh electronegative element is fluorine

§ Electronegativity by and large decreases every bit you motion downward a group and increases from left to correct across a menses § For representative elements, the values tend to increase from left to right across a menstruum § The trends that be amid these backdrop can be explained by variations in atomic structure
Chemistry Chapter 6 Periodic Table,
Source: https://slidetodoc.com/chemistry-chapter-6-the-periodic-table-and-periodic/
Posted by: warrenwifichaved.blogspot.com


0 Response to "Chemistry Chapter 6 Periodic Table"
Post a Comment